Tylenol (Paracetamol) and Autism: Is this a Hidden Factor in Rising Diagnoses?
For those of us in the functional and integrative health space, the recent HHS and White House announcement focusing on understanding the root causes of disease is welcome news. This seems to be a shift away from symptom management and toward a more comprehensive view of health has massive implications, especially for complex conditions like Autism Spectrum Disorder (ASD).
We are finally moving beyond the search for a single cause of autism. Instead, research points to a "whole-body" condition involving a dynamic interplay between genetics, the immune system, metabolic function, and the gut microbiome.
This evolving understanding is incredibly hopeful. By focusing on these interconnected systems, we can explore new, personalised interventions aimed at improving the health and quality of life for individuals on the spectrum. This is just the beginning, but it signals a powerful and positive change in direction. The implications of this for Pharma are also enormous, I expect the floodgates of Class Action Lawsuits will open.
White House Autism Action Plan
White House/HHS announcement: what was said, and why it matters
On 22 September 2025, the White House and HHS announced a package of autism-related actions.
Including:
(1) Support for Leucovorin (folinic acid) in children with cerebral folate deficiency who present with autism-like symptoms;
(2) New safety communications on acetaminophen/paracetamol use in pregnancy; and
(3) Fresh NIH funding to study genetic–environmental interactions.
Officials also said they would review aspects of vaccine manufacturing, administration, and timing (e.g., spacing), though scientific bodies continue to state there is no causal link between routine vaccines and autism. This article only touches on post vaccine tylenol and risk and does not discuss Vaccines in depth (that is a whole blog series on it’s own). (HHS.gov)
The first part of this article looks at some common question and answers, continue reading for more information. The links mentioned here are only a portion of the research and does not assume all of these factors are involved in every autism case.
Q&A
-
Understanding the definition of Autism Spectrum Disorder and the context behind its changing prevalence is of strategic importance for public health. This knowledge is crucial for allocating educational and healthcare resources, planning for community support, and demystifying public concern surrounding the sharp increase in diagnoses over the past four decades.
Autism Spectrum Disorder (ASD) is a neurodevelopmental condition characterised by persistent deficits in social communication and interaction, alongside the presence of restricted, repetitive patterns of behaviour, interests, or activities. It is a "spectrum" condition because its symptoms and severity vary widely, meaning every individual with ASD has a unique profile of strengths and challenges.
Over the last 40 years, diagnosis rates have climbed dramatically.
• In the 1980s, autism was estimated to affect approximately 1 in 2,000 children.
• By 2000, this figure had risen to 1 in 150.
• By 2020, U.S. prevalence estimates reached approximately 1 in 36.
This surge has fueled a significant debate between two primary arguments: whether the increase is due to improved recognition or a true increase in incidence.
• The "Improved Recognition" Argument: A large portion of the rise can be attributed to expanded diagnostic criteria, which now include milder forms of the condition that were previously uncategorised (such as Asperger's syndrome). Greater public and clinical awareness, coupled with better and more widespread screening practices, has also led to more children being identified. A striking 2011 study in South Korea, which actively screened a general population of schoolchildren, found a prevalence of 1 in 38, with the crucial detail that two-thirds of the cases had been unrecognised in the community. The authors concluded not that autism is more common in Korea, but that it is likely more common everywhere than previously thought, with many cases going unrecognised without active screening.
• The "True Increase" Argument: Proponents of this view note that the timeline of the diagnostic surge parallels certain shifts in our modern environment that began in the late 1980s. These include the widespread replacement of aspirin with acetaminophen for pediatric fevers during the 1980s and increased exposure to environmental chemicals. While correlation does not equal causation, these parallels have prompted hypotheses that environmental factors may be contributing to a genuine rise in the number of cases.
The current expert consensus holds that while broadened diagnostic criteria and heightened awareness are responsible for a substantial part of the increase, a genuine rise in incidence—driven by complex gene-by-environment interactions—is also plausible. To explore these interactions, it is essential first to understand the baseline genetic risk for the condition.
-
Genetics plays a central role in susceptibility to Autism Spectrum Disorder. Quantifying this genetic contribution helps researchers understand the condition's high heritability and provides the necessary foundation for investigating how environmental factors might interact with an individual's underlying genetic predispositions.
ASD is a complex disease with a high heritability index, involving multi-genetic changes. To date, over 800 genes have been associated with the condition, highlighting its genetic complexity. A large-scale, population-based study in Sweden provided robust estimates of this genetic influence. The study found that the heritability of ASD was 0.50 (50%), meaning that half of the variation in risk within the population could be explained by genetic factors. For the narrower, more severe diagnosis of Autistic Disorder (AD), the heritability was slightly higher at 0.54 (54%).
The same study quantified familial risk using the Relative Recurrence Risk (RR) metric, which compares the risk of ASD in a relative of an affected individual to the risk in the general population. The findings demonstrate a clear pattern where the risk increases with the degree of genetic relatedness:
• Monozygotic (identical) twins: 153.0 times the risk
• Full siblings: 10.3 times the risk
• Dizygotic (fraternal) twins: 8.2 times the risk
• Half-siblings (maternal or paternal): 2.9–3.3 times the risk
• Cousins: 2.0 times the risk
These results confirm that genetics create a significant diathesis, or predisposition, for ASD. However, with heritability estimated at 50%, it is clear that genetic vulnerability alone is not deterministic; environmental factors are critical in modulating whether that underlying risk manifests as a clinical diagnosis. One specific genetic variation that has been studied in this context is the MTHFR polymorphism.
-
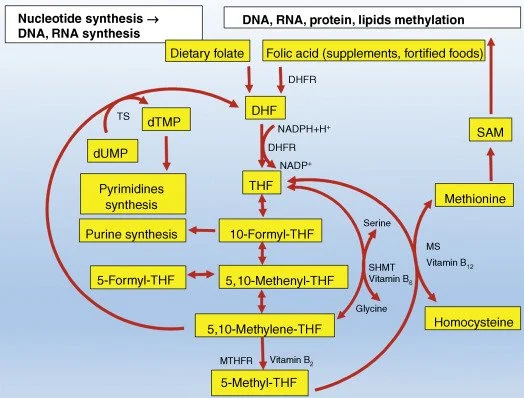
Investigating specific genes like MTHFR is vital for understanding the biological pathways that may be disrupted in ASD. The MTHFR gene is a key component of the folate metabolic pathway, which is essential for fundamental cellular processes like DNA synthesis and repair. Variations in this gene are therefore a critical area of research for their potential role in neurodevelopment.
The MTHFR gene provides the instructions for making an enzyme called methylenetetrahydrofolate reductase. This enzyme plays a critical role in processing folate (vitamin B9) into its active form, which is necessary for a wide range of metabolic functions. The MTHFR C677T polymorphism is a common and specific variation in this gene that can reduce the enzyme's efficiency.
Several large meta-analyses have explored the link between this polymorphism and ASD risk:
• A meta-analysis focused on the Chinese Han population concluded that the MTHFR C677T polymorphism is significantly correlated with an increased risk of autism. This association was particularly strong in the Northern Han subgroup.
• Another broader meta-analysis found that the C677T polymorphism was significantly associated with ASD susceptibility in Caucasian populations.
The implications of these findings are profound. They strongly suggest a gene-environment interaction where the MTHFR C677T genetic variation confers a risk for ASD that can be modulated by the availability of folate in the diet. This finding was a pivotal moment in ASD research, shifting focus from a purely neurological model to one that incorporates metabolic pathways and nutritional science, illustrating how ASD is a "whole-body" condition. This highlights how maternal health and nutrition during pregnancy can influence developmental outcomes.
-
The prenatal environment is a period of intense and vulnerable fetal neurodevelopment. A mother's overall health, nutritional status, and exposure to certain substances can profoundly shape her child's developmental trajectory, potentially increasing or decreasing the risk for conditions like ASD.
Maternal Body Mass Index (BMI)
A meta-analysis of seven studies revealed a clear link between maternal BMI and ASD risk. Compared to mothers with a normal weight, those who were overweight had a 28% higher risk and those who were obese had a 36% higher risk of having a child with ASD. The analysis also found a linear dose-response relationship, with a 16% increase in risk for each 5 kg/m² increment in maternal BMI.
Key Nutrients
• Vitamin D: Often called the "sunshine vitamin," Vitamin D is crucial for brain development. The "sunshine vitamin" hypothesis is supported by geographical data showing that global ASD prevalence correlates with latitude, with higher rates found in regions farther from the equator where sunlight is less intense. Multiple studies have confirmed that maternal vitamin D deficiency during pregnancy is associated with a significantly higher risk of having a child with ASD.
• Folate: As discussed with the MTHFR gene, folate is critical for neurodevelopment. Maternal supplementation with methylfolate or folinic (not folic acid)—especially for mothers who carry MTHFR mutations
Medication Use (Acetaminophen)
A systematic review of 16 highly eligible studies found a consistent association between prenatal acetaminophen use and adverse neurodevelopmental outcomes, including ASD and ADHD. The association was stronger with long-term use and appeared to be dose-responsive, meaning higher exposure was linked to greater risk.
Maternal Trauma
A mother's own life experiences can also be a factor. One study found that a mother's adverse childhood experiences (ACEs) are linked to more negative outcomes in her children diagnosed with ASD or ADHD. Interestingly, a similar association was not observed for the father's ACEs, suggesting a unique maternal influence.
The establishment of a healthy internal environment for the fetus is critical, and this biological connection continues at birth with the establishment of the infant's gut microbiome.
-
The gut-brain axis, the biochemical signaling network between the gastrointestinal tract and the central nervous system, is a rapidly emerging field of neuroscience. Investigating the connection between the gut microbiome and ASD is strategically important, as it may reveal new biological markers and therapeutic targets that are potentially modifiable through diet or other interventions.
A core observation in this field is that individuals with ASD often experience gastrointestinal issues and have a measurably different composition of gut bacteria compared to neurotypical individuals.
A groundbreaking study applied a machine learning method to a sibling-controlled dataset to identify a core set of bacteria linked to ASD. This analysis pinpointed a "robust microbiome signature" of 26 bacterial taxa. To confirm the finding, the researchers then successfully validated this signature across two completely independent cohorts of unrelated children, distinguishing them from controls with high accuracy (an average Area Under the Curve, or AUC, of 81.6% in the original dataset).
An infant's initial microbial colonisation begins prenatally and is heavily influenced by the mode of delivery. Recognising the microbiome's role has led to the exploration of several therapeutic approaches aimed at regulating it:
1. Additive Therapy: Using beneficial microbes (probiotics) to supplement the gut.
2. Subtractive Therapy: Using targeted antimicrobial agents, such as bacteriocins, to remove specific pathogenic bacteria.
3. Modulatory Therapy: Using diet, exercise, and other lifestyle changes to restore a healthy microbial balance.
The evidence indicates a strong, reproducible association between the gut microbiome and ASD, making it one of the most promising areas for future research and intervention. The influence of delivery mode on this microbiome also raises a related question about its independent role in ASD risk.
-
With Caesarean section (C-section) rates rising globally, understanding any potential association with neurodevelopmental outcomes has become a critical public health priority. A growing body of evidence suggests a link between mode of delivery and subsequent risk for ASD.
• A recent meta-analysis of previous studies had already reported that babies born via C-section had a 23% increased risk of being diagnosed with ASD compared to those born vaginally.
• These findings were reinforced by a much larger, multi-national cohort study of nearly 5 million births across five countries. This extensive analysis found a 26% higher risk of ASD in children delivered by C-section.
Importantly, the multi-national study found that this increased risk was consistent for births occurring between 36 and 42 weeks of gestation. Furthermore, there was no significant difference in ASD risk between children born via emergency C-section and those born via planned C-section.
Researchers have proposed several possible explanations for this association:
1. The underlying maternal or fetal conditions that necessitated the C-section may themselves be independent risk factors for ASD.
2. The difference in the infant's initial microbial exposure is significant, as vaginally born babies are colonized by their mother's vaginal and gut flora, while C-section babies are first exposed to microbes from the hospital environment and skin.
3. The timing of the birth, as C-sections are often scheduled before the full 40-week term, may interrupt a final, critical phase of neurodevelopment.
These factors highlight the influence of the immediate perinatal environment. Research is also expanding to consider the broader, ambient environment, including exposure to man-made fields.
-
This topic, while controversial, is gaining scientific attention due to its biological plausibility. The rapid proliferation of wireless technologies since the late 20th century has created an unprecedented change in our electromagnetic environment. This makes it a subject worthy of rigorous investigation for its potential biological effects, especially during sensitive developmental windows like pregnancy and early childhood.
The hypothesis is supported by several lines of reasoning and evidence:
• Correlation: The explosion in environmental exposure to electromagnetic fields (EMFs) and radiofrequency radiation (RFR) began in the late 1980s and 1990s, a timeline that runs parallel to the steep rise in ASD diagnoses.
• Proposed Biological Mechanisms: EMF/RFR is theorised to impact biology through several pathways that have been demonstrated in laboratory studies. Key mechanisms include:
◦ Oxidative Stress: EMF exposure can increase the production of reactive oxygen species (ROS), or free radicals, which are unstable molecules that can damage cells, proteins, lipids, and DNA.
◦ Mitochondrial Damage: Mitochondria, the cell's energy producers, can be damaged by EMF/RFR, leading to inefficient energy production and further cellular stress.
◦ Calcium Channel Disruption: EMFs may interfere with voltage-gated calcium channels, which are essential for communication between neurons.
◦ Blood-Brain Barrier (BBB) Permeability: Studies have shown that EMF/RFR can cause the protective barrier surrounding the brain to become "leaky," potentially allowing harmful substances to enter.
• Pathophysiological Overlap with ASD: There is a striking similarity between the documented biological effects of EMF/RFR exposure and the common pathophysiological abnormalities found in individuals with ASD. These include oxidative stress, mitochondrial dysfunction, immune dysregulation, and compromised BBB integrity.
• Epidemiological Evidence: A meta-analysis examining the effects of EMF exposure found an association with an increased risk of foetal developmental disorders, childhood cancer, DNA damage, and altered antioxidant parameters in offspring.
The fact that EMF exposure is hypothesised to damage mitochondria and increase oxidative stress provides a direct conceptual bridge to biomedical interventions, many of which are designed to support precisely these compromised biological systems.
-
The goal of biomedical and nutritional interventions is not to "cure" autism, but rather to address underlying biological issues such as metabolic, nutritional, or immune dysfunctions. By improving an individual's overall health and reducing distressing physiological symptoms, these treatments can enhance their quality of life and ability to benefit from established behavioral and educational therapies.
Folinic Acid (Leucovorin) DO NOT CONFUSE WITH FOLIC ACID
One of the most well-researched interventions targets a condition known as Cerebral Folate Deficiency (CFD), which has been identified in a subset of individuals with ASD.
• Mechanism: CFD occurs when the transport of folate into the brain is impaired. A primary cause is the presence of Folate Receptor Alpha Autoantibodies (FRAA), which block the receptor responsible for this transport. The prevalence of these autoantibodies is high in the ASD population, with studies finding them in around 71% of cases.
• Treatment: Folinic acid (also known as leucovorin) is a bioactive form of folate that can bypass the blocked receptor and enter the brain.
• Evidence: A landmark randomized, double-blind, placebo-controlled trial, which designated verbal communication as its primary outcome measure, found that high-dose folinic acid treatment led to significant improvements in children with ASD. The positive effects were most pronounced in the children who tested positive for FRAA.
Other Vitamins and Supplements
Several other nutrients are used to support key biological pathways, based on evidence of deficiencies or dysfunction in ASD. Methylfolate maybe helpful but it is important to assess whether someone is an OVER or UNDER methylator.
• Vitamin B6 and Magnesium: While formal studies show mixed results, this combination remains in clinical use based on the rationale that Vitamin B6 is an essential cofactor for neurotransmitter synthesis, and a subset of individuals may respond.
• Vitamin B12 (Methylcobalamin): This active form of B12 is used to support methylation pathways—a core biochemical process often found to be underactive in ASD—with clinical reports of improvements in language and attention.
• Omega-3 Fatty Acids: Commonly found in fish oil, these fats are known to help reduce inflammation and in some ASD cases may improve hyperactivity.
Dietary and Gut-Focused Approaches
Given the known link to gut health, interventions like probiotics and specialised diets (such as the gluten-free, casein-free diet) are often used. These approaches have shown anecdotal success, particularly in subgroups of individuals with significant GI symptoms or specific food sensitivities.
These interventions reflect a growing "whole-body" perspective on autism, where addressing underlying medical and nutritional issues can lead to meaningful improvements in both core and associated symptoms. Further clues to the biology of ASD can be found in its fundamental epidemiological patterns, such as its skewed sex ratio.
-
Do individuals with ASD experience trauma, and how does it affect them?
Recognising the intersection of Autism Spectrum Disorder and trauma is crucial for accurate diagnosis and effective care. Individuals with ASD are at a higher risk of experiencing adverse life events and trauma—including bullying, social rejection, and abuse—but the resulting symptoms are often missed or misattributed, leaving significant distress unaddressed.
A key challenge in this area is a phenomenon known as "diagnostic overshadowing." This occurs when symptoms of post-traumatic stress—such as hyperarousal, anxiety, or feelings of detachment—are mistakenly attributed to the core features of autism itself. For example, a state of hypervigilance from trauma may be misinterpreted as sensory hyperreactivity, and social withdrawal due to trauma may be seen simply as an autism-related deficit in social reciprocity.
Individuals with ASD may be more susceptible to the psychological impact of adverse events for several reasons:
• Sensory Hyperreactivity: An already sensitive nervous system can amplify the impact of a stressful event.
• Social and Emotional Processing: Difficulties with social understanding and emotional regulation can make it harder to process and recover from negative experiences.
• Differences in Sense-Making: Events that might be merely unpleasant for a neurotypical person can be experienced as profoundly upsetting or genuinely traumatic by someone with ASD.
This heightened vulnerability helps explain the strong association between adverse events and the high rates of co-occurring anxiety and depression in the autistic population. Due to the challenges of diagnostic overshadowing and communication differences, trauma-related symptoms in people with ASD are frequently underdiagnosed and, consequently, undertreated.
-
At The Barefoot Healers, we know how overwhelming this kind of information can feel. That’s why we take a holistic viewpoint — looking at genetics, nutrition, detox pathways, the microbiome, stress, and environmental factors — to help parents make sense of the bigger picture. We can guide you in exploring the best course of intervention, from safe fever management to nutritional support, while giving direction on supportive steps that nurture your child’s whole health and development.
Take advantage of our free discovery call to discuss your options https://l.bttr.to/wePJh
Introduction: The Expanding Puzzle of Autism
The rise in autism diagnoses over the past few decades has been striking. In the 1980s, autism spectrum disorder (ASD) was thought to affect about 1 in 2000 children; by 2020, that figure was closer to 1 in 36, according to The White House and HHS, the figures could be as closer to 1:10 or even worse (especially in boys).
While improved awareness and broader diagnostic criteria are major factors in this increase, the sheer scale of the change has led scientists to look beyond genetics alone. The conversation is shifting from a simple "genes vs. environment" debate to a more complex understanding of "gene-environment interplay."
Researchers are increasingly viewing autism not just as a condition confined to the brain, but as a whole-body condition influenced by a constellation of factors, from gut health to maternal nutrition. This article explores seven of the most surprising and impactful scientific takeaways that are reshaping our understanding of autism's origins.
-
Grosvenor, L.P., Croen, L.A., Lynch, F.L., Marafino, B.J., Maye, M., Penfold, R.B., Simon, G.E., Ames, J.L. (2024). Autism Diagnosis Among US Children and Adults, 2011–2022. JAMA Network Open, 7:e2442218. doi: 10.1001/jamanetworkopen.2024.42218.
Maenner, M.J., Shaw, K.A., Baio, J., Washington, A., Patrick, M., DiRienzo, M., Christensen, D.L., Wiggins, L.D., Pettygrove S., Andrews J.G., et al. (2020). Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years—Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2016. MMWR Surveill. Summ. 69:1–12. doi: 10.15585/mmwr.ss6904a1.
Zeidan, J., Fombonne, E., Scorah, J., Ibrahim, A., Durkin, M.S., Saxena, S., Yusuf, A., Shih, A., Elsabbagh, M. (2022). Global prevalence of autism: A systematic review update. Autism Res. 15:778–790. doi: 10.1002/aur.2696.
1. A Common Painkiller Is Under Scrutiny
The Surprising Link Between Acetaminophen aka Paracetamol/Tylenol and Neurodevelopment
A systematic review analysing 16 different studies found a "consistent association" between a mother's use of acetaminophen (the active ingredient in Tylenol/ Paracetamol) during pregnancy and adverse neurodevelopmental outcomes in her children, including an increased risk for ASD and ADHD symptoms.
This association appears to be stronger with long-term use and in a "dose-response fashion," meaning the risk increases with the dose. Researchers note that the replacement of aspirin (because of Reye’s Syndrome) with acetaminophen for children's fevers in the 1980s aligns temporally with the rise in autism rates. However, it's crucial to remember, as we are so often told……that correlation is not causation, and some sources refer to this as an "unproven link," highlighting the need for more conclusive research.
Scientists are exploring several potential biological mechanisms for this connection, including acetaminophen's impact on the endocannabinoid system, its disruption of maternal hormones, and its inhibition of the COX-2 enzyme. Given how commonly this painkiller is used, this research underscores the need for careful consideration of any medication use during pregnancy.
The Acetaminophen Conundrum: Oxidative Stress, Vulnerability, and the Question of Fever Suppression
Acetaminophen (AP), widely known as paracetamol or Tylenol, is generally regarded as safe; however, its increasing prevalence in maternal and infant use has raised hypotheses regarding its possible role in Autism Spectrum Disorder (ASD).
The basic biological function of AP is achieved by lowering hypothalamic prostaglandin E₂ (PGE₂) levels through the inhibition of Cyclooxygenase (COX) enzyme activity at the peroxidase site within the central nervous system (CNS), resulting in a lower temperature set-point and pain relief. The supposed mechanism linking AP to increased ASD risk, particularly when used repeatedly or in biochemically vulnerable individuals, revolves around two core physiological processes: detoxification and inflammation.
Mechanism: Glutathione Depletion and Oxidative Stress
When the body processes acetaminophen, it is detoxified in the liver via pathways that significantly consume glutathione, which is the body's primary antioxidant. Excessive or repeated AP use can therefore deplete glutathione and subsequently increase oxidative stress.
Research suggests that increased oxidative stress and inflammation are pathological mechanisms contributing to autism. Furthermore, the creation of the toxic AP metabolite, N-Acetyl-p-benzoquinone imine (NAPQI), has been hypothesised to affect neurodevelopment through excess formation in the brain.
Other proposed mechanisms include AP's effect on immunologic pathways that could disrupt microglia development and increase susceptibility to neurodevelopmental disorders, or disruption of the endocannabinoid system (ECS), which is important for neurodevelopment and analgesia. Long-term prenatal exposure to AP has even been associated with altered DNA methylation in cord blood samples of children diagnosed with ADHD.
The Context of Fever and Vaccination
The role of fever suppression, particularly post-vaccination, has come under scrutiny because the rise in AP use in infants (starting in the early 1980s) coincided with the rising rates of autism diagnoses. Vaccination generates a inflammatory immune response. The hypothesis suggests that if a child is already susceptible (e.g., due to impaired methylation capacity, lower glutathione reserves, or underlying mitochondrial disease), the combination of vaccine-induced inflammation and AP-induced glutathione depletion might lead to neural damage or disrupted development in the susceptible brain.
Some observational analyses found that babies given AP after the MMR vaccine had more autism risk than those given ibuprofen. However, it is essential to emphasise that correlation is not causation. As a precautionary measure, current expert advice recommends avoiding prophylactic antipyretics (pre-dosing for vaccines), and instead suggests treating distress after vaccination or during illness. Guidelines recommend using paracetamol or ibuprofen only if the child appears distressed, and not just for the sake of lowering the thermometre number.
-
Khan, et al. (2022). A Systematic Review of the Link Between Autism Spectrum Disorder and Acetaminophen: A Mystery to Resolve. PMC9385573.
Avella-Garcia, C.B., Julvez, J., Fortuny, J., et al. (2016). Acetaminophen use in pregnancy and neurodevelopment: attention function and autism spectrum symptoms. Int J Epidemiol. 45:1987–1996. doi: 10.1093/ije/dyw115.
Ji, Y., Azuine, R.E., Zhang, Y., et al. (2020). Association of cord plasma biomarkers of in utero acetaminophen exposure with risk of attention-deficit/hyperactivity disorder and autism spectrum disorder in childhood. JAMA Psychiatry. 77:180–189. doi: 10.1001/jamapsychiatry.2019.3259
Bauer, A.Z., Kriebel, D., Herbert, M.R., Bornehag, C.G., Swan, S.H. (2018). Prenatal paracetamol exposure and child neurodevelopment: a review. Horm Behav. 101:125–147. doi: 10.1016/j.yhbeh.2018.01.003.
Cited by the systematic review (as part of the underlying literature) for discussing potential mechanisms of prenatal exposure.
Vlenterie, R., Wood, M., Brandlistuen, R.E., et al. (2016). Paracetamol use during pregnancy and neurodevelopmental outcomes in the Norwegian Mother and Child Cohort Study. Int J Epidemiol. 45:2004–2008. doi: 10.1093/ije/dyw181.
Cited as a study categorizing AP use as short-term (1–27 days) and long-term (28 days and more) and measuring psychomotor development and temperament.
Shoffner, J.B., Trommer, A., Thurm, C., Farmer, W.A., Langley, L., III, Soskey, A.N., Rodriguez, P., D’Souza, S.J., Spence, K., Hyland, S., et al. (2009). Fever Plus Mitochondrial Disease Could Be Risk Factors for Autistic Regression. J. Child Neurol. 25:429–434. doi: 10.1177/0883073809342128.
Genetics, Detox Pathways, and Why CYP450 Matters
Genes, Folate, and Detox Pathways: Why Processing Matters More Than Intake
When it comes to autism risk, genes do matter, but they don’t tell the whole story. There are two levels to think about:
Genetics: the fixed DNA code you inherit from your parents. This includes single-gene mutations, common variations (SNPs), and larger DNA changes.
Epigenetics: the “switches” that turn genes on or off, influenced by diet, toxins, stress, infection, and lifestyle. Epigenetics can explain why one child with a genetic vulnerability develops autism while another with the same variant does not.
Autism is best understood as a gene–environment interplay: inherited vulnerabilities create the foundation, and environmental factors, nutrition, toxins, infections, medications, decide how those vulnerabilities are expressed.
MTHFR, CYP450, and Paracetamol
One of the most studied genes in autism is MTHFR, which controls folate metabolism and methylation. Methylation is critical for DNA repair, neurotransmitter balance, and making glutathione, the body’s master antioxidant.
Paracetamol (Tylenol) is mostly detoxified safely, but about 5–10% is processed through CYP2E1, creating a toxic byproduct (NAPQI) that can only be neutralised by glutathione.
If a child has an MTHFR SNP (like C677T or A1298C), their ability to make glutathione may already be lower. This also might be lowered by other medications.
If at the same time, CYP450 enzymes are induced (by drugs like rifampicin, phenytoin, or even smoking), more NAPQI is created. If they’re inhibited (by fluconazole, ciprofloxacin, fluoxetine, grapefruit juice), clearance slows and toxins linger.
This is why paracetamol can become riskier in children with MTHFR or detox pathway vulnerabilities, it tips an already strained system into oxidative stress.
Folate Biology: Why Folic Acid Isn’t the Same for Everyone
Folate (vitamin B9) is essential for brain development. Fortifying flour and cereals with folic acid has prevented thousands of neural tube defects worldwide. But for many people, folic acid isn’t the perfect solution.
Up to 40% of the population have MTHFR SNPs (C677T, A1298C), reducing their ability to convert folic acid into active methylfolate.
Unmetabolised folic acid can accumulate, blocking folate transport and disrupting methylation. They know this, so why is all our flour fortified?
Some children also develop Cerebral Folate Deficiency (CFD), often linked to Folate Receptor Alpha Autoantibodies (FRAAs). Children with autism are 19× more likely to test positive for FRAAs than typical peers.
A solution is folinic acid (leucovorin), which bypasses receptor blockages. In trials, it improved verbal communication, attention, and repetitive behaviours, especially in children with FRAAs.
Beyond MTHFR: Other Genetic Markers in Autism
Autism is not caused by one gene but by many different genetic patterns. These fall into three main categories:
1. Rare Single-Gene Mutations
Synaptic genes (make brain cell connections): SHANK3, NLGN, CNTNAP2, NRXN
Brain development genes: CHD8 (neuronal growth, organisation), DYRK1A (brain size, development)
Other rare genes linked to autism and intellectual disability: ARID1B, ASH1L, CHD2, POGZ, SYNGAP1
2. Common Variations (Polygenic Risk)
Small, common SNPs across many genes can add up to increase autism risk.
A parent may carry one SNP without symptoms but pass on a “risk load” that becomes significant in their child.
Key examples: MTHFR (C677T, A1298C), COMT (Val158Met), CBS (C699T, A360A), SOD2 (Ala16Val), GSTM1/GSTT1 null variants, MTR/MTRR polymorphisms.
These SNPs affect methylation, detoxification, oxidative stress, and neurotransmitter balance.
3. Copy Number Variants (CNVs)
Larger deletions or duplications of DNA.
Well-studied CNVs linked to autism include 16p (deletions and duplications) and 15q13.3 deletions.
4. Other Genetic Factors
Oxytocin Receptor Gene (OXTR): variations may affect social bonding and empathy.
Creatine Transporter Gene (SLC6A8): mutations disrupt energy metabolism, sometimes linked to autism-like syndromes.
Which SNPs May Worsen Risk?
Some SNPs may not cause autism on their own but can magnify vulnerability when combined with environmental stressors:
MTHFR (C677T, A1298C): impairs methylation, lowers glutathione → worsens oxidative stress from paracetamol or toxins.
COMT (Val158Met): slows dopamine breakdown → stress, anxiety, and methyl donor depletion.
CBS (C699T, A360A): speeds up homocysteine breakdown, lowering methyl donors and raising ammonia.
SOD2 (Ala16Val): weakens mitochondrial antioxidant defence → higher oxidative damage.
GSTM1 / GSTT1 null variants: reduced detox capacity → more vulnerable to heavy metals, pesticides, or medication byproducts.
CYP2E1 variants: alter paracetamol metabolism → more NAPQI, faster glutathione depletion.
Why This Matters
Autism risk is not about one mutation or cause, it is the stacking of risks. A child with MTHFR, COMT, and GSTM1 null variants may handle everyday exposures very differently from one without these SNPs. Layer on paracetamol, fortified folic acid, environmental toxins, or repeated infections, and the tipping point is reached sooner.
At The Barefoot Healers, we use Nordic Labs DNA profiling to identify these SNPs and other markers. Understanding this landscape allows us to recommend safer fever management, the right form of folate, antioxidant support, and a personalised plan that works with your child’s biology, not against it.
-
CYP450 enzymes are your body’s chemical “detox workers.” They help break down drugs, hormones, and toxins. If they’re slowed down (inhibited) or sped up (induced), detox balance changes—and this can influence how safe or harmful medicines like paracetamol are.
-
If you have MTHFR variants, your body may struggle to make enough glutathione. Since paracetamol uses glutathione for detox, this can leave you more vulnerable to oxidative stress, especially if CYP450 pathways are also induced or blocked.
-
Inhibitors (slow detox): fluconazole, ciprofloxacin, fluoxetine, grapefruit juice.
Inducers (speed detox and increase toxic byproducts): rifampicin, phenytoin, St John’s Wort, alcohol, smoking.
For a full list see here https://en.wikipedia.org/wiki/List_of_cytochrome_P450_modulators
-
Not necessarily. But if your child has MTHFR or detox vulnerabilities, repeated or high doses may carry more risk. It’s important to explore alternatives, support glutathione production, and avoid risky drug combinations. It has been suggested if you have to use paracetamol, then NAC and Glutathione may have protective effects
-
Yes. DNA profiling through Nordic Labs can identify MTHFR and related pathways. In some cases, pharmacogenomic panels also reveal CYP450 enzyme variants. This can guide safer medication choices.
-
Paracetamol is not the only option. Herbs (e.g., elderflower, yarrow, chamomile), tepid sponging, hydration, and rest can be supportive. Paracetamol or ibuprofen should be reserved for when the child is genuinely distressed, rather than automatically suppressing every fever. Always consult medical advice if you have a child with a president or very high fever.
-
Rossignol, D.A. (2021). Cerebral Folate Deficiency, Folate Receptor Alpha Autoantibodies and Leucovorin (Folinic Acid) Treatment in Autism Spectrum Disorders: A Systematic Review and Meta-Analysis. J. Pers. Med. 11:1141. doi: 10.3390/jpm11111141.
Supports the prevalence rates of FRAAs and Cerebral Folate Deficiency (CFD) in ASD, stating that children with ASD were 19.03-fold more likely to be positive for a FRAA compared to typically developing controls.
Li, Y., Qiu, S., Shi, J., Guo, Y., Li, Z., Cheng, Y., Liu, Y. (2020). Association between MTHFR C677T/A1298C and susceptibility to autism spectrum disorders: a meta-analysis. BMC Pediatr. 20:449. doi: 10.1186/s12887-020-02330-3.
Supports the association between MTHFR polymorphisms and ASD susceptibility.
Frye, R.E., Sequeira, J.M., Quadros, E.V., James, S.J., Rossignol, D.A. (2012). Cerebral folate receptor autoantibodies in autism spectrum disorder. Mol Psychiatry. 18:369–381. doi: 10.1038/mp.2011.175.
Supports findings on cerebral folate receptor autoantibodies (FRAAs) in ASD.
Mohammad, N.S., Jain, J.M., Chintakindi, K.P., Singh, R.P., Naik, U., Akella, R.R. (2009). Aberrations in folate metabolic pathway and altered susceptibility to autism. Psychiatr Genet. 19:171–176. doi: 10.1097/YPG.0b013e32832cebd2.
Supports the concept of aberrations in the folate metabolic pathway affecting ASD susceptibility.
Pu, D. et al. (2013). Association between MTHFR Gene Polymorphisms and the Risk of Autism Spectrum Disorders: A Meta-Analysis. Mol Autism, 4(13).
Frye, R. E. et al. (2018). Folate Receptor Alpha Autoantibodies in Autism Spectrum Disorders: Diagnosis and Treatment With Leucovorin. Molecular Psychiatry, 23, 247–256.
James, S. J. et al. (2004). Metabolic Endophenotype and Related Genotypes Are Associated With Oxidative Stress in Children With Autism. Am J Med Genet Part B, 131B(1), 60–68.
Hein, D. W. (2006). Molecular Genetics and Function of NAT1 and NAT2: Role in Aromatic Amine Metabolism and Carcinogenesis. Mutat Res, 612(2), 64–69.
Zhou, S. F. (2009). Polymorphism of Human Cytochrome P450 2E1 and Its Clinical Significance. Drug Metab Rev, 41(2), 110–120.
Rossignol, D. A. & Frye, R. E. (2014). Evidence Linking Oxidative Stress, Mitochondrial Dysfunction, and Inflammation in the Brain of Individuals With Autism. Front Physiol, 5, 150.
3. The Gut-Brain Axis is More Than a Buzzword
A "Microbial Signature" for Autism May Exist
Just as the body's ability to use nutrients is critical, so too is the complex ecosystem living within it, further demonstrating how autism's roots extend far beyond the brain. The gut-brain axis, the biochemical signaling that takes place between the gastrointestinal tract and the nervous system, is a major focus of modern health research. In the context of autism, a groundbreaking study used a machine learning approach called recursive ensemble feature selection (REFS) to analyze the gut bacteria of children with ASD and their neurotypical siblings.
The results were powerful: researchers identified a "robust microbiome signature" of just 26 specific bacterial types that could distinguish children with ASD from controls. The model achieved high accuracy across three independent groups of people, with an area under the curve (AUC) of over 80% for the best-performing classifiers. This suggests a strong, reproducible link that is not simply due to differences in lifestyle or diet.
This connection to the microbiome may begin at birth. The gut bacteria of babies born via Caesarean section are "dramatically different" from those born vaginally. C-section babies tend to have more hospital-associated microbes, such as Klebsiella, while vaginally born babies get most of their early bacteria from their mother. This difference may be significant, as a meta-analysis found that C-section delivery was associated with a 23% increased risk of ASD.
-
A large study identified a signature of 26 specific gut bacteria that can reliably distinguish children with Autism Spectrum Disorder (ASD) from their peers. The findings showed that children with autism tend to have lower levels of beneficial bacteria such as Bifidobacterium, Butyricicoccus, and Eubacterium eligens (important for making short-chain fatty acids like butyrate), and higher levels of certain strains of Clostridium, Parabacteroides, Sarcina, and others that may produce inflammatory or disruptive metabolites. This pattern, called dysbiosis, was reproducible across three different groups of children with over 80% accuracy.
-
The gut and brain communicate constantly through the gut–brain axis. A loss of protective bacteria means less butyrate, which normally supports gut lining health, reduces inflammation, and helps regulate neurotransmitters. An overgrowth of certain Clostridia and other species may increase toxins or immune activation that can affect brain development. While this doesn’t mean the microbiome causes autism, it shows that children with ASD often share a distinct gut profile that could become a target for future therapies through diet, probiotics, or microbiome-focused interventions.
-
Peralta-Marzal, L.N., Rojas-Velazquez, D., Rigters, D., Prince, N., Garssen, J., Kraneveld, A.D., Perez-Pardo, P., Lopez-Rincon, A. (2024). A robust microbiome signature for autism spectrum disorder across different studies using machine learning. Scientific Reports, 14, 814.
This is the primary source detailing the machine learning study. It supports the use of a machine learning approach, Recursive Ensemble Feature Selection (REFS), the finding of a robust set of 26 bacterial taxa (ASVs) that serve as a signature for ASD classification, and the high accuracy achieved across cohorts (average AUC of 81.6% in the sibling-controlled discovery dataset and 0.84 in validation datasets).
Cryan, J.F., Dinan, T.G. (2012). Mind-Altering Microorganisms: The Impact of the Gut Microbiota on Brain and Behaviour. Nat. Rev. Neurosci. 13:701–712. doi: 10.1038/nrn3346.
Supports the general concept of the microbiota–gut–brain axis and the communication pathways involved.
Ding, H.T., Taur, Y., Walkup, J.T. (2017). Gut microbiota and autism: Key concepts and findings. J. Autism Dev. Disord. 47:480–489. doi: 10.1007/s10803-016-2960-9.
Supports the general concept of the gut-brain axis in ASD and the fact that gastrointestinal problems and differences in microbial communities are commonly reported in ASD.
Dominguez-Bello, M.G., Costello, E.K., Contreras, M., et al. (2010). Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc. Natl. Acad. Sci. USA. 107:11971–11975. doi: 10.1073/pnas.1002601107.
Supports the claim that the delivery mode profoundly influences initial microbial colonization, showing that vaginally delivered infants acquire microbiota resembling the vaginal and perineal/fecal sources.
Shao, Y., Forster, S.C., Tsaliki, E., et al. (2019). Stunted microbiota and opportunistic pathogen colonization in caesarean-section birth. Nature. 574:117–121. doi: 10.1038/s41586-019-1560-1.
Supports that C-section delivery disrupts maternal-to-infant microbiome transfer. It also supports that C-section babies harbor higher levels of potential opportunistic pathogens in their guts, including Klebsiella spp..
Zhang, T., Sidorchuk, A., Sevilla-Cermeño, L., et al. (2019). Association of Cesarean Delivery With Risk of Neurodevelopmental and Psychiatric Disorders in the Offspring: A Systematic Review and Meta-analysis. JAMA Netw. Open. 2:e1910236. doi: 10.1001/jamanetworkopen.2019.10236.
Supports the finding from a meta-analysis that C-section delivery was associated with increased odds of autism spectrum disorders (Odds Ratio 1.33) and ADHD (Odds Ratio 1.17) compared with vaginal delivery.
Yip, B., Leonard, H., Stock, S., et al. (2016). Caesarean Section and Risk of Autism Across Gestational Age: A Multi-National Cohort Study of 5 Million Births. Int. J. Epidemiol. 46:429–39. doi: 10.1093/ije/dyw336.
Supports the strong epidemiological evidence linking C-section delivery to an increased risk of ASD (the basis for the claim of a 23% increased risk from a meta-analysis).
Noor, R., Maniha, S., Taniya, M. (2020). Cesarean Section Delivery and the Autism Spectrum Disorder: Risk and Consequences in Bangladesh. Biomed. Biotechnol. Res. J. 4:2115. doi: 10.4103/bbrj.bbrj_134_19.
Explicitly provides the figure that "a child born via C-section delivery has a 23% risk of developing ASD compared with a child born via vaginal delivery" based on a current meta-analysis study.
Kang, D-W., Adams, J.B., Coleman, D.M., et al. (2017). Microbiota Transfer Therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: An open-label study. Microbiome 5:10. doi: 10.1186/s40168-016-0225-7.
Supports the context that interventions targeting the gut can improve ASD symptoms (mentioned in the surrounding discussion about the microbiome importance and FMT efficacy).
Gershon, M. (1999). The Enteric Nervous System: A Second Brain. Hosp. Pract. 34:31–52. doi: 10.3810/hp.1999.07.153.
Supports the reference to the complexity of the GI tract, stating that the extensive network of nerve cells in the gut is considered the "second brain"
4. A Mother’s Health—and Her Own Childhood—Matters
The Echoes of a Mother's Life and Health
The child's internal ecosystem is seeded at birth, but it develops within the mother's own biological environment, connecting the child's risk to the mother's lifelong health. Recent meta-analyses have established a direct, dose-response link between maternal body mass index (BMI) and ASD risk. Children born to mothers who are overweight have a 28% higher risk of ASD, and for mothers with obesity, the risk is 36% higher. The data shows that the risk increases by 16% for each 5 kg/m² increment in maternal BMI.
Even more surprisingly, a mother's own childhood experiences appear to play a role. A recent study found that a mother's adverse childhood experiences (ACEs), such as abuse or neglect, are linked to more negative outcomes in her children who have neurodevelopmental disorders like ASD or ADHD. Interestingly, the study did not observe a similar link for fathers' childhood trauma. These findings broaden the concept of prenatal health, suggesting that a mother's lifelong metabolic status and even her past psychological trauma can be contributing factors.
-
Wang, Y., Tang, S., Xu, S., Weng, S., Liu, Z. (2016). Maternal Body Mass Index and Risk of Autism Spectrum Disorders in Offspring: A Meta-analysis. Sci Rep. 6:34248. doi: 10.1038/srep34248.
This meta-analysis provides the specific dose-response data: pooled relative risk (RR) of ASD was 1.28 (28% higher risk) for children born to overweight mothers and 1.36 (36% higher risk) for children born to obese mothers. It also established the linear dose-response relationship of a 16% increased risk for each 5 kg/m² increment in maternal BMI.
Gardner, R.M., Lee, B.K., Magnusson, C., et al. (2015). Maternal body mass index during early pregnancy, gestational weight gain, and risk of autism spectrum disorders: Results from a Swedish total population and discordant sibling study. Int J Epidemiol. 44(3):870-883. doi: 10.1093/ije/dyv081.
Supports the link between maternal BMI and ASD risk using a large cohort study.
Krakowiak, P. et al. (2012). Maternal metabolic conditions and risk for autism and other neurodevelopmental disorders. Pediatrics 129, e1121–e1128, 10.1542/peds.2011-2583.
Supports the idea that maternal metabolic conditions, including obesity, are risk factors.
Challier, J.C. et al. (2008). Obesity in pregnancy stimulates macrophage accumulation and inflammation in the placenta. Placenta 29, 274–281, 10.1016/j.placenta.2007.12.010
University of Gothenburg, Maria Davidsson. (2025). Mother’s Childhood Trauma May Worsen Child’s Autism or ADHD, Study Finds.
This is the primary source for the claim. It reports that a study found mothers’ adverse childhood experiences (ACEs), including abuse, neglect, and household dysfunction, are linked to negative outcomes in their children with neurodevelopmental disorders such as ADHD and autism.
University of Gothenburg, Maria Davidsson. (2025). Mother’s Childhood Trauma May Worsen Child’s Autism or ADHD, Study Finds.
Supports the finding that no similar association was observed for fathers, and discusses the mechanism that a mother's unsafe environment or difficult childhood affects her ability to manage stress and provide security, impacting the child's ability to regulate emotions.
Abdelrazek, M. & Rice, F. (2021). Maternal stress in pregnancy and child ASD: a genetically informed design. BJPsych Open (Abstract).
Supports the general link that maternal stress in pregnancy (which can be caused by trauma) may have a direct effect on ASD traits in offspring.
Leppert, B., Havdahl, A., Riglin, L., et al. (2019). Association of maternal neurodevelopmental risk alleles with early-life exposures. JAMA Psychiatry. 76:834–842. doi: 10.1001/jamapsychiatry.2019.0774
5. Sunshine and Latitude Are Unexpected Clues
The Vitamin D Hypothesis: A Link to Sunlight and Geography?
Beyond a mother's personal health history, broader environmental factors that shape her biology also appear to matter. One of the most interesting epidemiological patterns in autism research is a geographical one. Global studies show that autism prevalence tends to vary with latitude: rates are generally lower in countries nearer the equator and higher in regions farther from it.
This trend has given rise to the Vitamin D hypothesis. Sunlight is our primary source of Vitamin D, which is not just a vitamin but a potent "neurosteroid" essential for brain development and immune regulation during pregnancy. A growing body of evidence supports this theory.
A study in Sweden found that mothers who were deficient in Vitamin D during pregnancy had about double the odds of having a child diagnosed with ASD. A 2020 meta-analysis confirmed this, concluding that maternal Vitamin D status is "inversely associated with autism risk." The biological mechanism is plausible: Vitamin D regulates hundreds of genes in the developing brain and helps prevent inflammation that could be harmful to the fetus.
This hypothesis may also help explain certain demographic observations. For example, children of dark-skinned mothers living in northern latitudes have been observed to have higher rates of autism. Because melanin in the skin blocks UVB radiation, dark-skinned individuals require more sun exposure to produce adequate Vitamin D, putting them at higher risk for deficiency in low-sunlight regions.
6. Our "Electrical" Environment May Play a Role
A Controversial Clue: Electromagnetic Fields (EMFs)
Beyond the quantifiable factors like genetics and maternal health, researchers are investigating invisible environmental influences, such as electromagnetic fields (EMF), particularly in the context of inherent biological vulnerability observed in individuals with Autism Spectrum Disorder (ASD). Exposure to EMF, including radio-frequency radiation (RFR) emitted by devices like cell phones, has been shown to induce measurable biological effects at the cellular level.
The leading hypothesis connecting EMF exposure to potential risk involves the mitochondrial energy system and oxidative stress. Research indicates that EMF exposure can disrupt the antioxidant defense system, leading to increased oxidative damage. This is particularly relevant as ASD has been associated with a Mitochondrial Energy-Deficient Endophenotype, characterised by metabolic imbalances and oxidative damage. Studies suggest that melatonin, which functions as a key antioxidant and can easily cross the blood-brain barrier, exhibits a protective effect against EMF-induced oxidative stress.
Crucially, the cellular targets of EMF overlap with systems already identified as dysfunctional in ASD. For instance, RFR exposure has been shown to enhance the release of calcium ions from neuroblastoma cells in culture. Abnormal calcium signaling is specifically recognized as a mitochondrial component of calcium signaling abnormality in autism. Furthermore, research has reported that EMF, specifically 900 MHz cell phone frequency radiation, induced a significant increase in glutamate levels in primary rat neocortical astroglial cell cultures. These findings suggest that the electromagnetic environment may act as a stressor, interacting with pre-existing metabolic and signaling vulnerabilities—such as those involving mitochondria and calcium regulation—to potentially contribute to neurodevelopmental disruption.
The link is far from proven, but the core hypothesis is that the rise in autism diagnoses since the 1980s has coincided with an explosion of microwave-frequency EMFs from wireless technologies. The theory suggests that chronic EMF exposure might interfere with critical biological processes during brain development.
The plausibility of this hypothesis comes from the significant overlap between the known biological effects of EMFs and the biological abnormalities frequently found in individuals with ASD.
Key parallels include:
• Oxidative Stress: EMF exposure is a known trigger of oxidative stress, a state of cellular damage from harmful free radicals. This is significant because oxidative stress is one of the most consistent biological findings in individuals with ASD, suggesting EMFs could amplify a core vulnerability.
• Altered Calcium Signaling: Studies have shown that EMFs can affect cellular calcium channels. These channels are critical for regulating neuronal excitability, a process thought to be imbalanced in ASD.
• Blood-Brain Barrier (BBB) Permeability: Some research indicates that EMFs can make the protective blood-brain barrier "leaky." Increased BBB permeability is also a feature of the neuroinflammation seen in some individuals with ASD.
A meta-analysis found that maternal EMF exposure was associated with a 1.34-fold increased odds of fetal developmental disorders.
Paradoxically, while chronic, ambient exposure to certain EMFs is under investigation as a risk factor, researchers are also exploring whether targeted, extremely low-frequency fields could be used therapeutically to modulate brain activity. A real nod towards understanding our inner and outer electromagnetic environment.
A pilot study used this approach as a treatment for children with ASD and found statistically significant improvements in vocabulary and reductions in anxiety and attention problems. This finding reinforces the idea that EMFs can modulate brain activity, but far more research is needed to understand the risks and potential applications.
7. The "Why More Boys?" Question Has a Fascinating Answer
The Gender Gap: The "Female Protective Effect"
After exploring a range of external risk factors, a final clue comes from within, revealing how an individual's innate biology can mediate their response to all these influences. One of the most consistent facts about autism is that it is diagnosed in approximately 3 to 4 boys for every 1 girl. The leading scientific theory to explain this disparity is not that boys are inherently more vulnerable, but rather that females appear to have a built-in biological protection.
This "Female Protective Effect" (FPE) hypothesis is supported by several lines of evidence. First, studies have shown that females with ASD tend to carry a larger number of autism-linked genetic mutations than affected males.
This suggests it "takes a bigger genetic hit" for a girl to cross the diagnostic threshold. Second, the non-autistic siblings of autistic girls show higher rates of autistic traits than the siblings of autistic boys. This implies that the entire family of an autistic girl likely carried a higher overall genetic and environmental risk load.
Taken together, these findings suggest that the biological threshold for developing autism is simply higher in females, requiring a more significant accumulation of risk factors to manifest. Understanding the biological basis of what protects females could provide powerful clues for developing future interventions.
Conclusion: A New Era of Understanding
Autism arises from a complex, dynamic interplay between genetic vulnerabilities and a wide array of environmental and biological factors. The scientific consensus is shifting from viewing ASD as a static, brain-only disorder to seeing it as a "whole-body" condition involving the gut, the immune system, and metabolism.
This growing knowledge is not just academic; it opens new doors for prevention and personalized intervention. The discovery of Folate Receptor Alpha Autoantibodies, for example, has led directly to a targeted and effective treatment—high-dose leucovorin—that can produce life-changing improvements in verbal communication for a subset of children. This proves that understanding these underlying biological factors can translate into tangible, hopeful outcomes. It empowers us to move beyond a one-size-fits-all approach and toward strategies that support healthier neurodevelopment for everyone.
As the saying in the autism community goes, "If you've met one person with autism, you've met one person with autism," a reminder of the condition's profound heterogeneity. The puzzle is far from complete, but each new piece brings us closer to a more comprehensive picture. It prompts a vital question: As science continues to connect the dots between our environment and our biology, what might a world designed for healthier neurodevelopment look like?